Welcome to PICU Doc On Call, A Podcast Dedicated to Current and Aspiring Intensivists.
I’m Pradip Kamat and I’m Rahul Damania. We are coming to you from Children’s Healthcare of Atlanta – Emory University School of Medicine.
I will turn it over to Rahul to start with our patient case…
A 2 yo Asian M presents with difficulty feeding. He has a history of epilepsy and recently was switched to Valproic Acid for seizure control as well as OTC deficiency diagnosed at birth. He has had a 3-day history of URI, cough, which now progressed to this difficulty feeding. His parents state he was initially very fussy however in the past few hours he has been more sleepy. He has not had any fevers. They have noticed that while he is sleeping he has been breathing “fast.” Prior to arrival at the emergency room, he was noted to have a large non-bloody, non-bilious emesis. Upon transfer to the trauma bay, the patient suddenly has a seizure. A quick POC glucose is normal. His care is escalated & diagnostic workup is initiated.
Pradip, our case had two key elements in his history, namely the h/o OTC deficiency & VPA use, which place him, particularly at high risk to have hyperammonemia. As this is our topic of discussion today, would you mind starting with a general background & definition of hyperammonemia?
Sure, this is a classic case of not only hyperammonemia but also a metabolic crisis in this case related to a urea cycle defect.
As background, the urea cycle is the metabolic pathway that transforms nitrogen to urea for excretion from the body. We get nitrogen sources from a few areas in the body:
- from peripheral (muscle)
- enteral sources (protein ingestion)
The urea cycle occurs in the liver and once the ammonia is converted to urea in the hepatocyte, it is excreted into the kidney as urea. We will dive into this deeper soon, however, pathologies that impair adequate hepatocyte function, can impair the urea cycle and thus lead to hyperammonemia.
This is a great basic science summary, would you mind commenting about this patient’s enzyme defect — the OTC deficiency?
- Yes, Ornithine transcarbamylase, or OTC for short, is one of the first few enzymes in the urea cycle.
- As a background, the inheritance pattern of majority, all of the urea-cycle-defects (UCD) is autosomal recessive, however, OTC deficiency is different — it is X linked.
- In a 21-year, multi-center retrospective study, it was noted that only 34 % of patients with UCD presented during the neonatal period (<30 days of age) — and around 25% of cases present in the 2-12-year-old range. This is why I would like to drive home this clinical point to have a urea cycle defect or any inborn error of metabolism in your differential, especially in a child who presents in a critically ill, undifferentiated state.
Why do you think there are subsets of populations who present later?
- This is a great question and the cause may be multi-factorial — it is worth noting that patients may have partial enzyme deficiencies and this may be a major reason why patients may have atypical presentations after the newborn period. This delayed presentation is most commonly seen in patients with partial ornithine transcarbamylase (OTC) deficiency.
As we have highlighted key pathophysiologic components, do you mind highlighting the typical clinical presentation of a child with a UCD & hyperammonemia?
The presentation may be variable, however, let’s break down some key features which were in our case:
- Patients typically have a preceding illness such as a URI or gastroenteritis, which triggers a more catabolic state.
- As a result, patients end up having increased ammonia levels — this ends up creating a picture of somnolence, inability to maintain normal body temperature, poor feeding, vomiting, and in severe cases lethargy, and This is a similar presentation to sepsis and thus keeping your differential broad, having fine attention to trends in vitals or clinical exam, and early aggressive management with contingency planning is crucial to the care of these patients.
As we wrap up the clinical presentation, what would be some other physical exam abnormalities we will see upon initial presentation?
I would like to highlight some important points here:
- Subtle signs of elevated ammonia include behavioral modifications such as delirium, as well as neuro-developmental delay — thus it is important to recognize our aforementioned presentations of seizures & alteration of consciousness
Let’s finish this episode with management pearls, Rahul, what is your general approach to hyperammonemia?
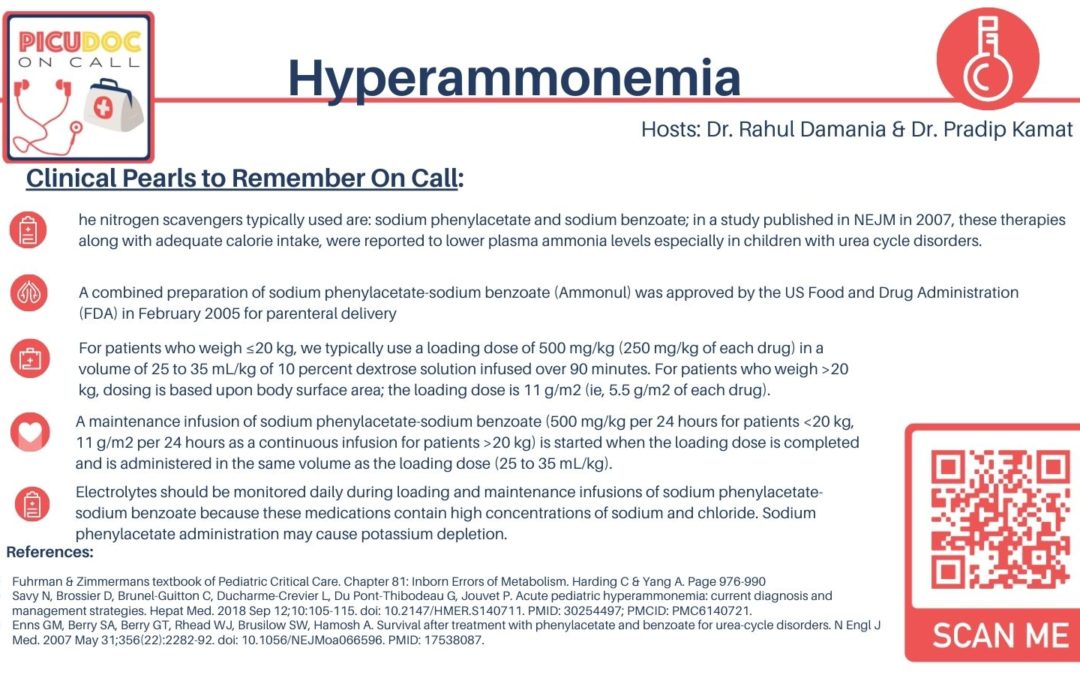
Excellent, the nitrogen scavengers typically used are: sodium phenylacetate and sodium benzoate; in a study published in NEJM in 2007, these therapies along with adequate calorie intake, were reported to lower plasma ammonia levels especially in children with urea cycle disorders. A combined preparation of sodium phenylacetate-sodium benzoate (Ammonul) was approved by the US Food and Drug Administration (FDA) in February 2005 for parenteral delivery
Any recommendations on dosing?
- For patients who weigh ≤20 kg, we typically use a loading dose of 500 mg/kg (250 mg/kg of each drug) in a volume of 25 to 35 mL/kg of 10 percent dextrose solution infused over 90 minutes. For patients who weigh >20 kg, dosing is based upon body surface area; the loading dose is 11 g/m2 (ie, 5.5 g/m2 of each drug).
- A maintenance infusion of sodium phenylacetate-sodium benzoate (500 mg/kg per 24 hours for patients <20 kg, 11 g/m2 per 24 hours as a continuous infusion for patients >20 kg) is started when the loading dose is completed and is administered in the same volume as the loading dose (25 to 35 mL/kg).
- Are there some adverse events that we need to watch for in our patients?
- Most of the side effects for Ammonul are metabolic (eg, hypokalemia, hyperchloremia, acidosis), neurologic (eg, seizures), or respiratory (eg, respiratory distress or failure).
- Electrolytes should be monitored daily during loading and maintenance infusions of sodium phenylacetate-sodium benzoate because these medications contain high concentrations of sodium and chloride. Sodium phenylacetate administration may cause potassium depletion.
Going back to the NEJM trial, for children who were treated with Ammonul with recurrent admissions for hyperammonemia, the overall survival which was reported was 84 percent. It is important to note however, the neurologic outcome was not evaluated.
As I review the urea cycle, I see that arginine and citrulline are precursors which can help form urea, can you comment on their role in hyperammonemia?
- IV arginine hydrochloride is used as part of the initial management of metabolic decompensation in all forms of UCD except known arginase deficiency.
- Arginine is created via the urea cycle and in our case, this patient has an OTC deficiency so Arginine now becomes an essential amino acid.
- Blood pressure should be monitored since high doses of IV arginine can decrease blood
What about citrulline?
- In OTC or CPSI deficiency, small oral doses of citrulline also are provided because incorporating aspartate nitrogen may improve clearance as urea.
- In one retrospective study, patients treated with L-citrulline reduced ammonia levels and improved weight gain which was most likely due to increased protein intake.
When you look longitudinally, and before we go into hemodialysis and its role, are there certain medications that we want to avoid?
- Glucocorticoids increase protein catabolism and should not be used routinely.
- As patients may have seizures, remembering that valproic acid inhibits urea synthesis, leading to increased serum ammonia levels. Thus, VPA should not be routinely used.
Seizures may be treated with other antiepileptic drugs, although correcting the underlying metabolic abnormality is more likely to affect seizure control.
- Yes, finally, Mannitol is ineffective in treating cerebral edema caused by hyperammonemia due to UCDs.
- Let’s conclude this episode with hemodialysis; whats the appropriate timing?
- Hemodialysis should be started as soon as possible after hospital admission of a patient with severe hyperammonemia. Indications include an ammonia level that is rapidly increasing, acute hyperammonemia that is resistant to initial drug therapy, and/or ammonia that is persistently above the range of 350 to 400 micromol/L.
As many of our centers have CVVH readily available, it is important to consult with your nephrology team to optimize flow rates to be >40 to 60 mL/min. This method is less desirable as an initial treatment, although it can be used effectively between hemodialysis treatments to continue removing ammonia.
What is our endpoint usually if we are to go down the HD or CVVH route?
- Ammonia concentration is measured hourly during dialysis.
- Hemodialysis is stopped when the ammonia concentration has dropped below 200 micromol/L because it appears to have little effect below this level.
- What is important to recognize though is that plasma ammonia may increase again (rebound) because of the delay in the effect of nitrogen scavenging medications and the ongoing catabolism.
- Thus, hourly monitoring of ammonia levels is continued until ammonia levels have stabilized below 200 micromol/L for at least 24 hours → after a while to decrease iatrogenic blood draws, the frequency of measurements can be reduced to every four hours.
To summarize today’s episode…
- In newborns, UCDs typically present after 24 to 48 hours of age. Clinical features include somnolence and poor feeding followed by lethargy, vomiting, and coma. Other features include central hyperventilation leading to initial respiratory alkalosis, hyperammonemia, and seizures.
- The initial laboratory evaluation for suspected UCD should include arterial pH and carbon dioxide; serum ammonia, lactate, glucose, electrolytes, and amino acids; and urine organic acids and orotic acid. Elevated plasma ammonia concentration combined with normal blood glucose and normal anion gap strongly suggests a UCD.
- The initial approach to the treatment of UCDs consists of volume repletion, ammonia removal, protein restriction, and stimulation of anabolism. Respiratory status must be closely monitored. Pharmacologic therapy for hyperammonemia consists of initial IV administration of a combination preparation of sodium phenylacetate-sodium benzoate (Ammonul) followed by maintenance with oral glycerol phenylbutyrate (Ravicti).
This concludes our episode today on Hyperammonemia. We hope you found value in this short podcast. We welcome you to share your feedback & place a review on our podcast. PICU Doc on Call is co-hosted by me and my cohost Dr. Rahul Damania. Stay tuned for our next episode!

why is mannitol not used in UCD ?